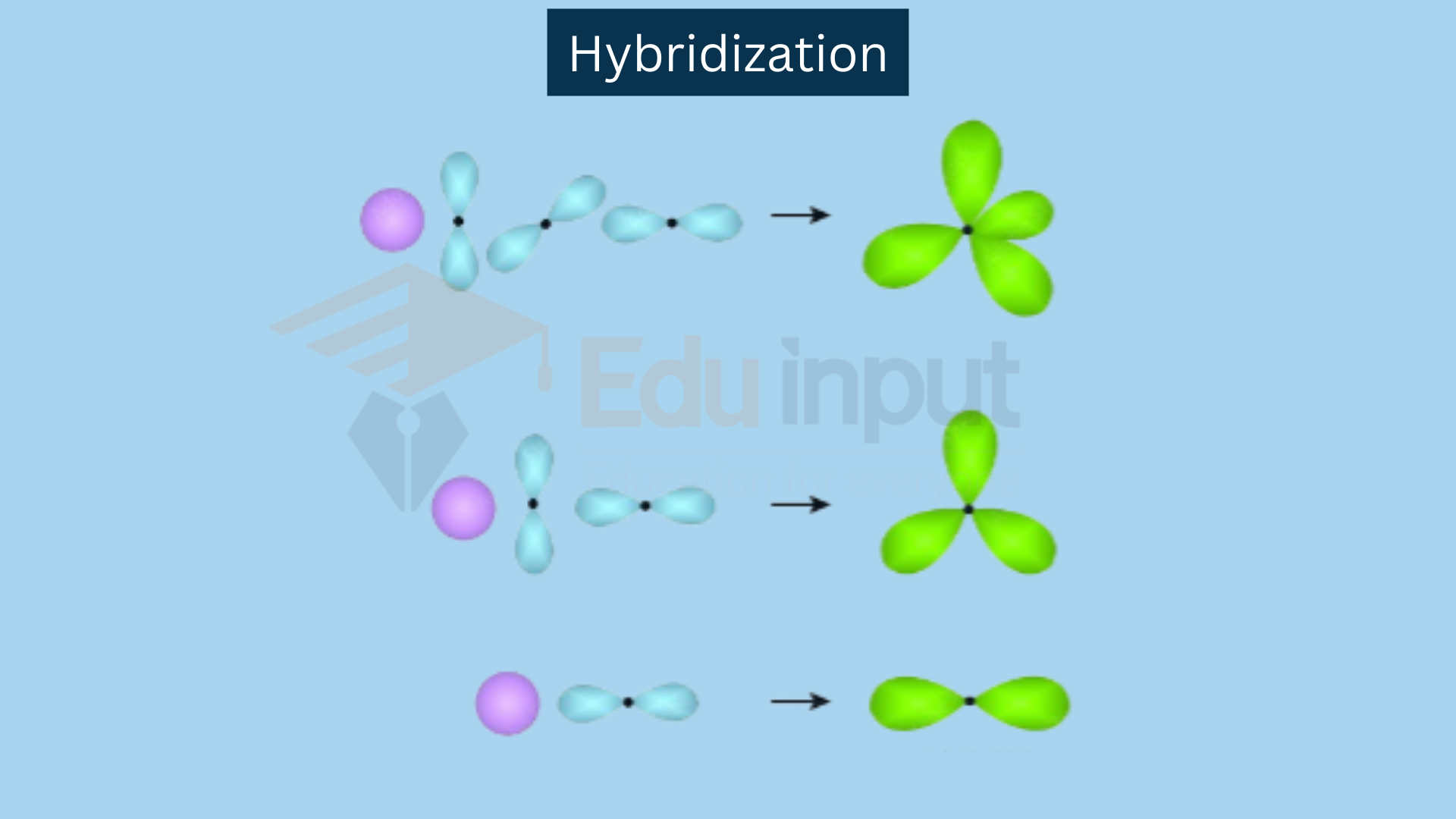
Hybridization Review . This organic chemistry video tutorial explains the hybridization of atomic orbitals. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. Energy changes occurring in hybridization. Now that we understand hybridization states, let's do a couple of examples, and.
from eduinput.com
In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. Energy changes occurring in hybridization. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Now that we understand hybridization states, let's do a couple of examples, and. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive.
Hybridization, definition, types and significance
Hybridization Review In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Energy changes occurring in hybridization. Now that we understand hybridization states, let's do a couple of examples, and. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. This organic chemistry video tutorial explains the hybridization of atomic orbitals. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,.
From www.adda247.com
What is Hybridization? sp3, sp2, Examples and Formula Hybridization Review Energy changes occurring in hybridization. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. This organic chemistry. Hybridization Review.
From www.scribd.com
Hybridization Review Worksheet PDF Chemical Bond Covalent Bond Hybridization Review Energy changes occurring in hybridization. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. Now that we understand hybridization states, let's do a couple of examples, and. Hybridization is a simple model that deals with. Hybridization Review.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk Hybridization Review Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Energy changes occurring in hybridization. In sp hybridization, one s orbital and one. Hybridization Review.
From www.youtube.com
Hybridization Theory YouTube Hybridization Review Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Now that we understand hybridization states, let's do a couple of examples, and. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Energy changes occurring in. Hybridization Review.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples Hybridization Review Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Now that we understand hybridization states, let's do a couple of examples, and. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization,. Hybridization Review.
From leah4sci.com
sp3, sp2, and sp Hybridization Review for MCAT Chemistry Hybridization Review This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. Now that we understand hybridization states, let's do a couple of examples, and. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2. Hybridization Review.
From www.pinterest.com
Microarray Comparative Genomic Hybridisation The procedure of Hybridization Review This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Hybridization of an s orbital with two p orbitals (p x and. Hybridization Review.
From www.masterorganicchemistry.com
What Are Hybrid Orbitals and Hybridization? Master Organic Chemistry Hybridization Review Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Now that we understand hybridization states, let's do a couple of examples, and. Hybridization is a simple model that deals with mixing orbitals. Hybridization Review.
From fity.club
Hybridization Chart Hybridization Review This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Now that we understand hybridization states, let's do a couple of examples,. Hybridization Review.
From www.studocu.com
Hybridization review Hybridization Review One “s” and 3 “p Hybridization Review This organic chemistry video tutorial explains the hybridization of atomic orbitals. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Energy changes occurring in hybridization. Now that we understand hybridization states, let's do a couple of examples,. Hybridization Review.
From www.youtube.com
Hybridization │ Review of Fundamentals │ Organic Chemistry YouTube Hybridization Review Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Energy changes occurring in hybridization. Hybridization of an s orbital with two. Hybridization Review.
From www.genome.gov
Hybridization Hybridization Review This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Now that we understand hybridization states, let's do a couple of examples, and. Hybridization of an s. Hybridization Review.
From www.youtube.com
Hybridization And Geometry With ConceptHow to Hybridization Review This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Energy changes occurring in hybridization. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. Now. Hybridization Review.
From www.genome.gov
Hybridization Hybridization Review This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Now that we understand hybridization states, let's do a couple of examples, and. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Energy changes occurring in hybridization. Hybridization of an s orbital with two p orbitals (p x. Hybridization Review.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Review This organic chemistry video tutorial explains the hybridization of atomic orbitals. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Now that we understand hybridization states, let's do a couple of examples, and. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2. Hybridization Review.
From studylib.net
Hybridization Hybridization Review This organic chemistry video tutorial explains the hybridization of atomic orbitals. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Energy changes occurring in hybridization. Now that we understand hybridization states, let's do a couple of examples, and. Hybridization of an s orbital with two p orbitals (p x and p y). Hybridization Review.
From www.pinterest.com
HYBRIDIZATION Organic chemistry, Teaching chemistry, Chemistry basics Hybridization Review In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. This is part of the valence bond theory and helps explain bonds formed, the length of bonds,. Energy changes occurring in hybridization. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Hybridization of an s orbital. Hybridization Review.
From www.youtube.com
SP2 Hybridization YouTube Hybridization Review In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals. This review discusses recent advances in our understanding of the evolutionary importance of ancient hybridization, adaptive. Hybridization is a simple model that. Hybridization Review.